Which One of the Following Systems Has the Highest Entropy
Which one of the following systems has the highest entropy. Which of the following systems has the highest entropy.

Which State Of Matter Has The Highest Entropy Study Com
For the second question the system with the lowest entropy is sugar crystals in 95 degrees centigrade cup of coffee.
. 10mL of water at 10C b. In which of these systems is the entropy decreasing. 1 mol of HCN c.
Which of the following has the highest entropy S. Indicate which of the following has the lowest standard molar entropy S. All have the same entropy because all are water.
H_2 g HBrO_4 g HBr g He g The SI units are Joules J for each of the following EXCEPT ___. T T T 1. The Meaning of Oxidation and Reduction.
Enthalpy Free energy Work Entropy. This problem has been solved. 2 kg of HCN d.
1 mol of HCN c. Explain your answer in a separate sheet. CH 3CH 2OH e.
Enthalpy Free energy Work Entropy. 10mL of water at 50C c. 10 mL of water at 10 C b.
One of the better ones that should be considered is the one in the following link because it is free to download. It will be maximum in gases followed by liquids and least in solids. Here hydrogen gas has more entropy as it shows more randomnessdisorderliness due to less molar mass than all the given substances and also in the gas phase.
Thus systems that are disorganized have greater entropy. Changing the pH of the solution that the mitochondria are in results in a similar change the pH of the intermembrane space. 05 g of HCN b.
A cup of ice at 0 degrees c a cup of water at 50 degrees c a cup of water at 0 degrees c a cup of ice at 15 degrees c. The Third Law of Thermodynamics is concerned with the limiting behavior of systems as the temperature approaches absolute zero. 2 kg of HCN d.
C Absolute Entropy Explanation. Once the system reaches the macropartition with the highest multiplicity highest entropy it will stay there. C Absolute Entropy d Absolute Free Energy.
H2Os at 50C b. Which one of the following systems has the highest entropy. Indicate which of the following has the highest entropy at 298 K.
In which of these systems is the entropy decreasingA. THIS SET IS OFTEN IN FOLDERS WITH. The entropy will usually increase when.
An isolated system being initially in a non-equilibrium state will evolve from macropartitions with lower multiplicity lower probability lower entropy to macropartitions with higher multiplicity higher probability higher entropy. Hence here the gas is only hydrogen and it has the highest entropy. For the first one the system with the highest entropy is a cup of water at 0 degress centigrade.
2 mol of HCN e. 2 mol of HCN e. CH 3CH 2OH e.
Which of the following has the highest entropy. Indicate which of the following has the lowest standard molar entropy S. If a system is left to change spontaneously in what state will it end.
This is the best answer based on feedback and ratings. Entropy is a measure of chaos or disorder in a system. 10mL of water at 100C d.
There are such systems on the market for phone management systems. Indicate which of the following has the highest entropy at 298 K. H2Og at 150C c.
G AS L iq u id. One can measure ATP production from isolated mitochondria in vitro in a test tube. A reaction occurs that results in an increase in the number of moles of gas.
All of the above have the same entropy at 298 K. 05 g of HCN b. Salt dissolving in water D.
Most thermodynamics calculations use only entropy differences so the zero point of the entropy scale is often not important. Air escaping from a tire B. A gas condensing to a liquid Weegy.
See the answer See the answer See the answer done loading. 10 mL of water at 50 C c. Entropy is a measure of disorder.
10 mL of water at 100 C d. 88 which one of the following systems has the highest. Which of the following would have the highest entropy assuming one mole of each substance and the same temperature for each.
Which of the following has the highest entropy S. A solid changes to a liquid. Entropy is defined as the amount of disorder within a system.
All have the same entropy because all our water. Solution for Which has the highest entropy. Hence option B is correct.
1 Show answers Another question on Chemistry. A molecule is broken into two or more smaller molecules. Chemistry 22062019 0720.
All of the above have the same entropy at 298 K. Which of the following has the highest entropy S.

Solved Which Of These Species Has The Lowest Entropy S Chegg Com

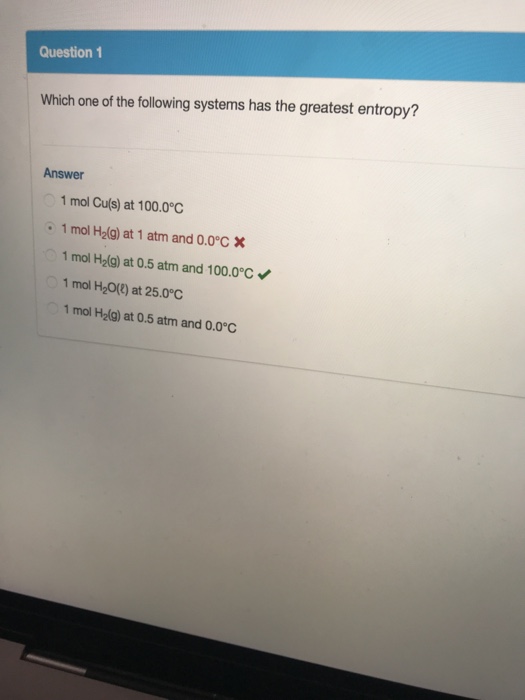
Solved Question 1 Which One Of The Following Systems Has The Chegg Com

Comments
Post a Comment